Custom Medical/Life Science Parts
Stamping, Sheet Metal Fabrication, and In-House Tooling Backed By Generations Of Metal Forming Experience.


Providing a complete medical device and life science parts manufacturing solution.
Delivering solutions to a demanding market like medical device parts is no small feat. It requires expertise in a range of manufacturing capabilities, including progressive stamping, sheet metal fabrication, fiber laser cutting, tooling, robotic welding, logistics, and much more. Plus strict quality management and compliance. Also needed is a team of skilled and experienced people dedicated to providing exceptional customer service. The professionals at Ajax understand these demands, and are committed to being the reliable, trusted partner medical OEMs require.
Ajax is an ISO 13485:2016 and ISO 9001:2015 certified metal forming solutions provider that has been in business since 1945.
The Coventor
A Medical Device Case Study

This comparably low-cost device, known as the “Coventor,” was designed by University of Minnesota Anesthesiology fellow Dr. Steve Richardson and medical device developers at the Earl E. Bakken Medical Device Center. Ajax provided medical device parts for this system.
As the spread of COVID-19 became intense during the spring of 2020, demand for medical device parts for ventilators at intensive care units became overwhelming. In response, University of Minnesota Anesthesiology fellow Dr. Steve Richardson came up with an idea for a simple ventilator that could be produced and delivered to the field quickly to help save lives. Dr. Richardson’s concept did not require pressurized oxygen or air supply, as did commercially available mechanical ventilators.
Dr. Richardson started work on his ventilator over a weekend, sourcing equipment and resources from biomedical engineer friends partnering with a product development team at the U of M’s Earl E. Bakken Medical Devices Center. Dr. Richardson and his team moved with extreme urgency, as the need for ventilators increased by the hour. The Ajax team offered to assist, and played a key role.
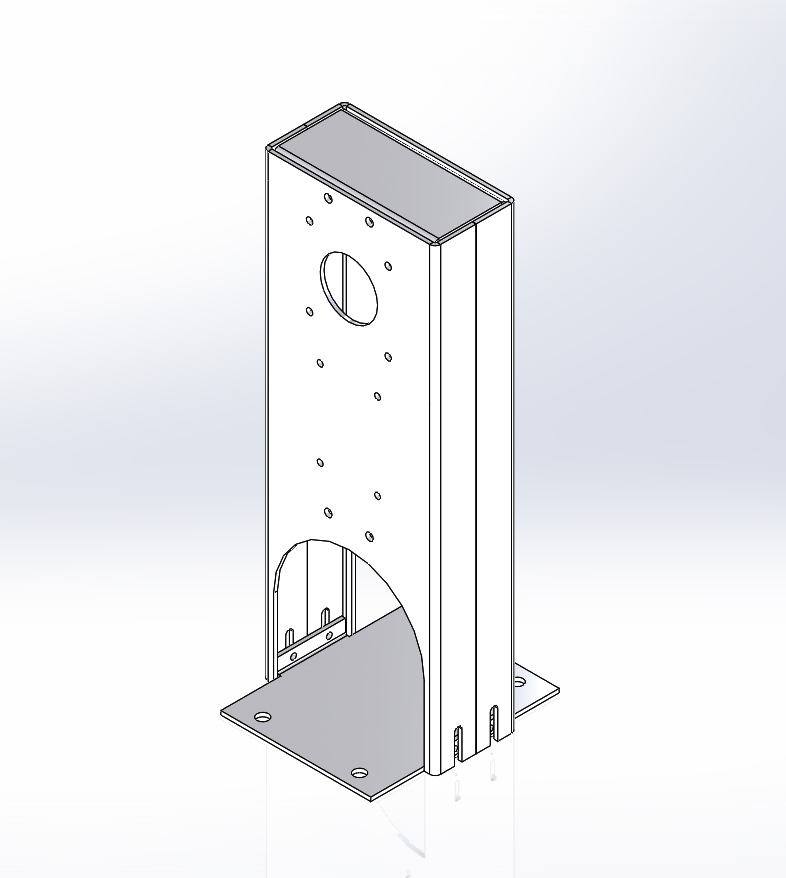
Essential to the unit was an enclosure. The developers selected aluminum for the enclosure, and made it compact and relatively inexpensive to manufacture and distribute. During the initial phases of development, prototypes were needed to begin testing.
Ajax stepped in to provide prototype enclosures virtually overnight, enabling the development team at the to move quickly to get the unit ready for actual use in the field.
FDA Production Approval
The US Food and Drug Administration authorized the production, use, and distribution of the device. UMN released the Coventor as open-source, letting companies interested sign a free electronic license and download the manufacturing specifications.
“From the outset, the mission of this project was to make this emergency device available to people in need, wherever they might be in the world, as quickly and safely as possible,” said Dr. Richardson, MD, a cardiac anesthesiology fellow in the Medical School, M Health Fairview.
“Through the tremendous hard work, ingenuity, and force of will of hundreds of individuals coming together as a team, we made that a reality in a matter of weeks.”
The Coventor has since been deployed to various locations around the world, including South America, Africa, and India.


